Agricultural Genomics Solutions
A platform suitable for all your research stages
Agrogenomics solutions from Affymetrix provide breeders and researchers with a powerful and flexible genotyping tool that cost-effectively identify, validate, and screen complex genetic traits in plants and animals.
Affymetrix's genetic analysis tools give you the ability to:
Discover
■Determine genetic diversity through genetic analysis technology
■Analysis of group structure
Related
■Identification of genetic markers related to ideal traits
■Confirm the mark-trait association
■Understand genetic adaptability to the environment
manage
■Use genetic information to select desired results
■Screening for ideal traits of plants and animals
Advantages of chip-based genotyping:
economy
■Cost-effective genotyping tools
Simple
■Combining multiple genotyping applications on a single technology platform
■Easy and simple process
■Accurate results within a few hours
flexible
■High-throughput genotyping tools are suitable for high-density or targeted genotyping applications
■Can genotypify all relevant markers of interest
■Low sample volume requirement
Chip-based genotyping products from Affymetrix provide complete solutions for applications ranging from genome-wide analysis to routine screening, with the highest accuracy and repetition, simple processes and lowest cost.
Axiom® genotyping solutions provide you with a variety of chips.You can choose custom content for the species you want to study, or select genotype-validated content from the Axiom® genomic database.
powerful
■ Genotype any species, any genomic size, and any ploidy level
■ Axiom® analysis detects insertions or deletions and ensures that all candidate SNPs are included, and can be up to 10 bp from adjacent SNPs, enabling more efficient QTL analysis
reliable
■ As low as 100 ng DNA, genotyping results are available for all sample types
■ Genotype detection rate ≥99%
Extended
■ Fully automated process that can handle up to 8 chip boards per week without adding manual or instrumentation
■ There are 96 or 384 samples on a chip board
■ Detect up to 2.6 million mutations per sample
Plant genotyping solutions
Automatic detection of polyploid and diploid genotypes without manual operation
Affymetrix works with scientists from academic research institutions and commercial seed companies to design chips for a variety of plants, including rice, wheat, corn, potatoes, tomatoes, cotton, soybeans, strawberries, and landscape plants.These chips allow researchers to identify genes associated with ideal phenotypic traits.
 | ■ Affymetrix has developed advanced genotype algorithms and software tools to automatically analyze non-diploid complex genomes ■ This algorithm provides tunable parameters to accurately type samples of inbred populations and genomes that deviate from reference sequences. |  |
Axiom® Corn Genotyping Chip ■ Currently the only genotyping chip that covers the SNP site of corn with high density, including 609,442 SNPs and 6,759 insertions/deletions. ■ These markers were obtained by screening Axiom® myDesign™ GenotypingArray containing 1.2 million SNP sites on 288 major corn samples from different lines of the world. ■ AffymetrixSNPolisher™ Analysis accurately typing SNPs. |  |
Axiom® Wheat Genotyping Chip ■ The highest coverage wheat genotyping chip.Using the 96 chip plate model, it cooperated with the University of Bristol to design for the global wheat strains, including 817,000 SNP sites, covering the entire wheat genome, greatly accelerating the modern wheat molecular breeding process. ■ Breeding researchers carefully selected polymorphisms in excellent wheat strain hexploids, including SNP markers of fragment contigs determined by the International Wheat Sequencing Association (IWGSC), with a total of 35,143 markers distributed in A and Band in the D genome. |  |
Axiom® Cotton Genotyping Chip Cotton genotyping chip contains 35,550 markers in total ■ 28,158 intraspecies-specific markers identified using gene enrichment sequence regions of upland cotton (G. hirsutum). ■ 7,392 markers found using genome simplification methods based on the conservation of restriction cleavage sites (GR-RSC). ■ 5,286 marks found during interspecies assembly of upland cotton (G. hirsutum) and island cotton (G. barbadense). ■ 2,016 specific markers in the species of upland cotton (G. hirsutum). ■ 380,000 custom marks can be added to the chip or polymorphic marks can be transferred to the Axiom® 384HT myDesign™ breeder chip with 100% fidelity to the The need for sample research and analysis of different groups. |  |
Axiom® Soybean Genotyping Chip A total of 180,961 markers were selected from 20 soybean chromosomes, representing wild species and cultivating species ■ 114,735 SNPs or 63.4% markers were located in 40,631 genes. ■ 22,952 SNPs are located within 13,259 regions upstream or downstream of the gene. ■ 43,274 SNPs are located in intergenic regions. ■ The discovery and verification of SNP was accomplished using a combination of 16 soybean varieties in South Korea and 31 soybean varieties in China. ■ This chip was evaluated by a combination of 228 lines, including high-deep resequencing lines, repeat DNA samples from different sources, repeat DNA samples, different cultivation and wild lines, as well as multiple F2 generation and recombinant inbred lines. |  |
Axiom® Strawberry Genotyping Chip Chip covers the entire genome of cultivated hybrid strawberries (Fragaria x ananassa) ■ 95,062 SNPs and insertion deletions from octaploid and diploid varieties, including: 1,761 complex alleles SNPs and 3,751 SNPs from diploid varieties. ■ Representing multiple strawberry varieties, a diverse set of global breeding germplasm resources has promoted the development of SNP. ■ SNP discovery, 9 octaploid varieties were sequenced and analyzed by more than 20 times coverage, including Holiday, Korona and F1 seedlings hybridized to Holiday and Korona; two possible diploid ancestors, Fragariamandschurica and F. iinumae;A known diploid variety, F. vesca sequencing data were aligned with F. vesca genome sequences. |  |
Axiom® Rose Genotyping Chip ■ Axiom® Rose Genotyping Chip (WagRhSNP Axiom Array) is designed through the Affymetrix® expert design project with the Plant Breeding Group of Wageningen University in the Netherlands and Germany Designed by the Institute of Plant Genetics, Leibniz University. ■ A total of 68,893 SNPs are selected from tetraploid fresh cut flower roses and garden rose varieties. ■ Research on complex traits in coping with roses: polyploid linkage map, identification of SNP haplotypes, and QTL analysis of important phenotypic traits. ■ 672 samples were genotyping validated using the Axiom Rose genotyping chip, including: tetraploid fresh cut rose population K5, tetraploid garden rose, ploidy levels from diploid (2x) to pentploid (5x) 13 varieties. |  |
| Axiom® myDesign™ genotyping custom chip Flexible, cost-effective genotyping custom chips Affymetrix provides economical genotyping custom chips for researchers individuals or collaborative groups.Work with our Bioinformatics team to design chips with relevant content for multiple applications, from discovery to inquiries. Consistent SNP content and fast turnaround time for each batch ■ Get 100% identical SNP content per order, as long as your research requires ■ No SNP loss - Consistent and flexible custom format every time the content on the chip is Flexible custom format ■ Can contain markers of multiple species on the same chip ■ Multiple analysis of 1,500-675,000 SNPs can be designed on each chip, which is cost-effective and allows you to obtain more information scalability Scalability ■ Low starting quantification of 480 samples can meet your budget ■ Order custom chips again with as low as 192 samples to complete your research |  |

Automatic detection of polyploid and diploid genotypes by software
Greatly accelerate your analysis process with professional bioinformatics support and simplified software
Strong Informatics Support
■ Axiom® software uses the statistical clustering prediction tools FitAllo and AxiomGT1 algorithm to accurately and flexibly distinguish genotypes, and
Detected polyploid and diploid genotypes
Integrate with your existing system
■ Highly automated options: Affymetrix® PowerTools (APT) based on command line
■ Seamless integration of third-party software packages
■ Compatible with 32-bit and 64-bit Windows® 7 and Windows Server 2008 operating systems
Simplified data analysis
■ Includes flexible SNP filtering and output tools, which can be output into PLINK format
■ Visualization tools include scatter plots, curve charts, and heat maps
■ The SNPolisher software package can automatically classify SNPs, making it easier for you to control genotypes (as shown in the figure below)

The most suitable platform for agricultural genomics projects
No sacrificing data quality and turnaround time
The rapid development of next-generation sequencing has helped agricultural scientists build a wide range of genome resources, which will create a "living world of genome libraries."Scientists, animal breeding experts and commercial seed companies have all begun to get involved in this huge genomic library resource, thereby strengthening agricultural genomics strategies.Identification and selection by applying genomic markers
Select important traits, their goal is to increase productivity and commercial viability.This technical guide integrates the experience of sequence-based genotyping methods presented in peer-reviewed journals and compares the performance of chips in agricultural genotyping applications to assist you in making decisions on genotyping techniques.
Overview of sequence-based genotyping
Genomic selection and association mapping or linkage imbalance (LD) localization techniques require a large number of markers to accurately estimate traits associated with genotypes.This requires that the technology used to obtain genotype information must be cost-effective and high-throughput.Whole genome sequencing and targeted genotyping using sequence capture are expensive, and low-throughput methods for generating genotype data are still unrealistic for conventional applications.With the goal of determining economical sequencing, sequencing-based genotyping methods such as simplified DNA sequencing based on enzyme digestion 1 (RADseq) and genotyping-by-sequencing (GBS) 2 have been continuously developed in scientific research and daily applications.The potential is constantly cited.
The sequencing-based approach relies on adding barcodes to multiple samples and reduces the cost of genotyping.This technique utilizes restriction enzymes to digest target restriction sites and low-copy genomic regions to reduce genomic complexity.This allows regions with repeat sequences to be avoided, which are prone to fuzzy or false SNPs and increase sequencing costs.The genotype data obtained using sequencing vary greatly in quality and quantity, which is highly dependent on the genome size and structure of the organism and the population evaluated.The complexity of genomic structures, such as ploidy levels, GC content and repeat sequences, genetic diversity of the population to be studied, and mating systems within the population, all have the cost, accuracy and efficiency of sequencing technology to accurately and easily collect genotype data.Direct impact.Sequencing-based genotyping techniques are useful for species with lagging or imperfect label exploration.Both GBS and RADseq can be used for at least 96 samples without access to the reference database or previously found markers.This technique is also particularly suitable for screening thousands of polymorphisms to understand the consequences of genetic variation, which have previously relied on extremely small numbers of markers such as microsatellites and amplified fragment length polymorphisms (AFLP).Genotyping techniques based on sequencing have been used for the discovery of markers.The results of various experiments conducted on multiple species have been published in the journal, such as barley, corn, wheat, cattle and trout.There are several reasons why using sequencing-based genotyping in conventional genotyping is still far away, and some of them are listed in this article.The special issue of Molecular Ecology magazine on genotyping-by-sequencing technology 3 also summarizes that the new genotyping-by-sequencing technology is still imperfect and cannot be fully expanded in different plants and animals.
Key experimental factors in sequence-based genotyping All next-generation sequencing platforms have limits on the number of sequence bases, and they are produced by each sequencing run.This limited output capability means that sequencing-based genotyping runs must balance four key parameters: level of sample multiple analysis, genomic coverage, sequence coverage, and cost per sample.Multiple analysis of samples is critical, because the sequencer's limited output capability must be shared by all samples included in the run.More samples mean fewer sequencing bases per sample. Genome coverage is also important because it determines the percentage of the genome being analyzed and, therefore, the number of markers available in the genome.Higher genome coverage is achieved at the expense of one of the other parameters, as it requires more sequencer output capability. |  |
Sequence coverage (or sequence "depth") determines the average number of reads per sequence in the dataset.In fact, some sequences are read frequently, while some are read less, or not at all.Sequence coverage affects the percentage of gaps in the data and genotype accuracy.Accurate genotype detection usually requires 30 times or higher coverage on each SNP.Increasing sequence coverage also forces compromise elsewhere to balance the use of sequencer capabilities.Of course, sample multiplexed analysis, genomic coverage and sequence coverage can all be improved by putting more operations on the sequencer, but this can increase costs rapidly.This technical guide discusses the impact of experimental methods in each novel sequencing technology, the impact of genomic complexity on the number of markers, and the scope of application.Changes in experimental methods can significantly increase the cost of any genotyping project, which may be five times more for 30,000-marked projects.
application
Genome coverage is highly dependent on the choice of genotyping techniques and methods, and this choice ultimately depends on the application of interest.Each method provides different levels of plant or animal genome coverage.This affects the number of markers available and also determines which approach is best for the target application.These applications range from population genome scanning to identifying phylogenetic development.Figure 2 shows how different sequencing and chip-based genotyping methods are localized to various applications, as well as the relative costs associated with covering the genome.The number of markers covered by each sequencing-based method depends on experimental parameters such as the type of restriction enzyme, the quality and quantity of DNA, and the analytical techniques.The number of markers for each application is shown in Figure 2, which is a function of the genome portion being sequenced.The number of markers required for accurate genotyping is a function of the number of linkage imbalances at the genome level, the recombinant events captured in the genealogy, and the divergence between groups.4 By changing the restriction enzymes in the assay to increase the number of tags, genome coverage can be improved.However, as noted above, increasing genome coverage comes at the expense of lower sample multiple analysis, lower sequence coverage, or higher cost per sample.

Population genome scanning and sequencing verification: Chips and sequencing-based genotyping techniques have been used to conduct population scanning and validate markers found using next-generation sequencing.Sequencing-based genotyping strategies are prone to detect false SNPs because of inherent errors in sequencing technology, copy number variations cannot be localized to the reference genome, or from paralogic or homologous genes.With deeper sequence coverage, false SNPs can be excluded, but this increases the cost per sample, which can also be avoided by using double haploid or high-quality reference sequences, but these can lead to more complexInformatics analysis, while strict filtering conditions discard most of the sequencing data.By running large quantities of samples in the population to verify the marks, large and duplicate marks can be identified.High-density Axiom® chips have been successfully used to validate sequencing discovery and exclusion of false SNPs, which are the result of sequencing errors in many species, including Chicken 5 and Salmon 6.The chip brings an easy way to evaluate millions of markers in different populations and verify those found through different sequencing technologies such as RADseq, RNAseq, and resequencing.
Associative mapping, genome selection and copy number application: Associative mapping (AM) technology uses a large number of polymorphic markers to overcome challenges and limitations in QTL localization.Association mapping relies on linkage imbalance and recombination present in existing genomic banks to conduct phenotype-genotype associations between random mating populations, lines, or germplasm.8 In association mapping studies, more markers increase the possibility of finding or localizing pathogenic variants, 9 Therefore, the more markers, the better.Although association mapping can be accomplished by sequencing-based genotyping methods, chips can often more cost-effectively genotyping high-density markers with better data quality and integrity.
Dense markers can also be used in genomic selection, where the effects of markers are simultaneously estimated in genotyping and phenotypic detection, or training populations, and then used to predict the value of the selection candidates.The accuracy of genome selection increases with the increase in marker density.It is estimated that 50,000 markers are enough to accurately predict these relationships.10 Copy number variation detection enables the study and identification of heritable variation in complex traits.
Genealogy and quantitative trait loci (QTL) mapping: Unlike association mapping, QTL mapping looks at the effects of multiple genes on quantitative traits, such as salmon controlling resistance to sea lice or the QTL of fish egg size.QTL identification is based on biparent hybridization, requiring the identification of single genes in chromosomal regions through fine mapping, so a large number of hybridizations are required to generate a sufficient number of meiotic events.Genealogy utilizes breeding materials in QTL assays, which cover multiple generations and are associated with common ancestors in the pedigree through multiple hybridizations.This enables the identification and use of most alleles present in breeding programs.
Phylogenetic and population positioning: The population structure may vary in the germplasm maintained by various research institutions and the germplasm maintained by breeders.Different population structures require different genome-wide association studies (GWAS) methods.Information on the rate of genomic recombination can be provided by constructing genetic or linkage maps to investigate population structure and conducting phylogenetic analysis.Understanding the population structure also helps to select appropriate markers and density.Population analysis can be completed by studying markers in a small portion of the genome.
Workflow: The workflow comparison between chip-based technology and sequencing-based genotyping technology is shown in Figure 3.Genotyping techniques based on sequencing rely on barcode techniques to perform multiple analysis of samples (e.g., sequencing 96 samples in a single channel would require 96 sample barcodes).Library preparation requires the selection of restriction enzymes suitable for the species and the number of labels required.This process requires optimization to avoid problems such as primer dimers, which may increase the cost of sequencing.After library preparation, the real sequencing takes about 11 hours to 11 days, depending on the capability of the instrument and the percentage of sequencing genome.Higher sample multiplexed analysis is also not impossible, but as mentioned earlier, genome size, percentage of sequencing genomes, and sequence coverage in a single channel must be balanced.After sequencing, the data are filtered and the barcodes are demultiplexed to extract the marks for each sample.Important considerations for using either genotyping technology are computing devices and analytical processes.The analysis process needs to be customized based on the species of interest, experimental methods, populations to be studied and the technology itself.
A recent article by James Hutton Institute 11 concluded that an important result of using GBS to study barley is that GBS data is more processed and subsequent analysis than the multiple SNP analytical techniques currently used in the lab.Challenging.There are many challenges in adopting sequencing-based genotyping techniques, including computing devices, bioinformatics experts who maintain customized analysis processes, software for comparing and analyzing, and the time it takes to extract useful genotyping data.Data analysis of sequencing technology is usually carried out in the "cloud" to minimize local data storage and computing requirements.Mutation detection is often carried out through customized software, which detects various genotypes.Each storage technology has costs associated with data transfer, storage and retrieval, which affects the cost of genotyping projects.
In contrast, chip-based genotyping technology makes it easy to genotype millions of data points per sample using desktop workstations, reducing equipment costs while improving operational efficiency.The simplicity and ease of use of this chip method makes the chip available in a variety of scenarios and environments, which is particularly attractive for conventional breeding applications, as large sample processing and turnover time are important.
DNA quality and quantity in library preparation: The requirements of sequencing-based genotyping technology on DNA concentration and DNA quality are still one of the serious challenges in practical applications.DNA sequencing requires several micrograms (μg) of purified high molecular weight genomic DNA without contamination and symbionts.Bacterial contamination may affect sequencing, as random amplification of DNA materials means fine
Bacterial DNA is sequenced with the biological sample to be typing.
Number and type of markers: The biggest difference between chip technology and sequencing technology lies in the ability of chips to target specific genomic regions or specific SNPs, as shown in Figure 4.Chip-based technology is able to target any number of markers within a specific chromosomal region, and its design strategy uses markers that are evenly spaced in the genome and, if necessary, use markers that are higher-spaced in a specific region of the genome.This flexibility allows the chip to be applied to GWAS, 12 QTL mapping, association mapping and genome selection, with a certain amount of definitive bias.By conducting SNP studies on multiple varieties, the determination bias can be reduced.

Table 1: The amount of DNA required to genotypify biological samples using chips and sequencing-based genotyping techniques.The concentration required for sequencing-based genotyping techniques is 2 to 30 times that of chips.

Genotyping techniques based on sequencing rely on random sampling of DNA libraries, and the number of markers is proportional to the number and size of the regions to be sequenced.When the restriction sites of the population to be studied are conserved, the genomic region is expected to be unbiased.Therefore, labeling between samples is not conservative and no two samples can provide the same set of labels.This results in data loss and requires
Complex informatics recovers lost data through inference.Unconserved markers between samples must be calculated by reference genome or by using very high coverage sequencing (18 times or higher) from haplotypes of related lines.
Considerations for choosing a sequencing method
For any of the applications mentioned above, various factors that affect genotyping must be taken into account before deciding which method to adopt.
Heterozygous detection errors: sequencing-based genotyping techniques, especially GBS, rely on low coverage to reduce costs and obtain a large number of markers that can be used for association mapping.The disadvantage of this experimental method is that the detection of heterozygotes is significantly too low, which affects the accuracy of genotypes.GBS detected less than 50% of heterozygotes.A study on the coverage required for DNA sequencing 14 predicted that for each heterozygous diploid, detecting two alleles at 99.75% of the loci at least once requires a 13.5-fold depth.While detecting each allele at least twice will require 18 times the depth.Increasing sequencing coverage results in higher cost per sample and makes sequencing more expensive than chips.Studies on grapes show that 30-50% of heterozygotes were not detected when genotyping at an average depth of 5.7 times.15 The accuracy of detection of heterozygotes on chip is determined by the chip design, which is highly predictable, making the accuracy of detection of genotypes close to 100%.Further design methods used in the chip enable genotype genomic regions with GC content above 60%.
Genome coverage: The number of markers brought by any technology is expected to achieve uniform coverage of the genome.Sequencing-based genotyping techniques exhibit data loss, which leads to uneven coverage of the genome.The lost data is caused by a function of experimental conditions and genomic structure, resulting from a combination of library complexity (i.e. the number of unique sequence tags) and library sequence coverage.The amount of lost data is directly related to the multiple levels of library preparation and the enzymes used for RE digestion.The selection of restriction enzymes in sequencing techniques affects allele signal loss, thus affecting population genetics statistics.Rare markers require infrequent cleavage of enzymes, which subsequently produce fewer markers.If frequent cleavage enzymes are used, more marks will be produced, but the coverage will be significantly reduced, resulting in a large amount of data loss.
All representative reduced sequencing techniques rely on reduced genome complexity, which reduces costs and increases throughput.The disadvantage of reduced complexity is that the genotype data obtained has obvious missing data.16 Genotype data may be lost due to inherent differences in genomic structures such as presence-deletion differences, variation in polymorphic restriction sites, and differential methylation, which affect the methyl used in representatively reduced sequencing techniques..Lost data is important for QTL mapping, where the quality of genotype data of parent lines is crucial for genotype detection of mapping populations.Parental lines need to be sequenced at very high coverage.
Figure 5: Explain the relationship between sequence coverage and missing data, a result published in a recent paper comparing sequencing-based genotyping on different platforms.17 This study shows that at 10 times the coverage, 1,000 marks can be obtained and 50% of the data are lost, while when the number of marks at low coverage increases to 30,000, 90% of the data is lost.
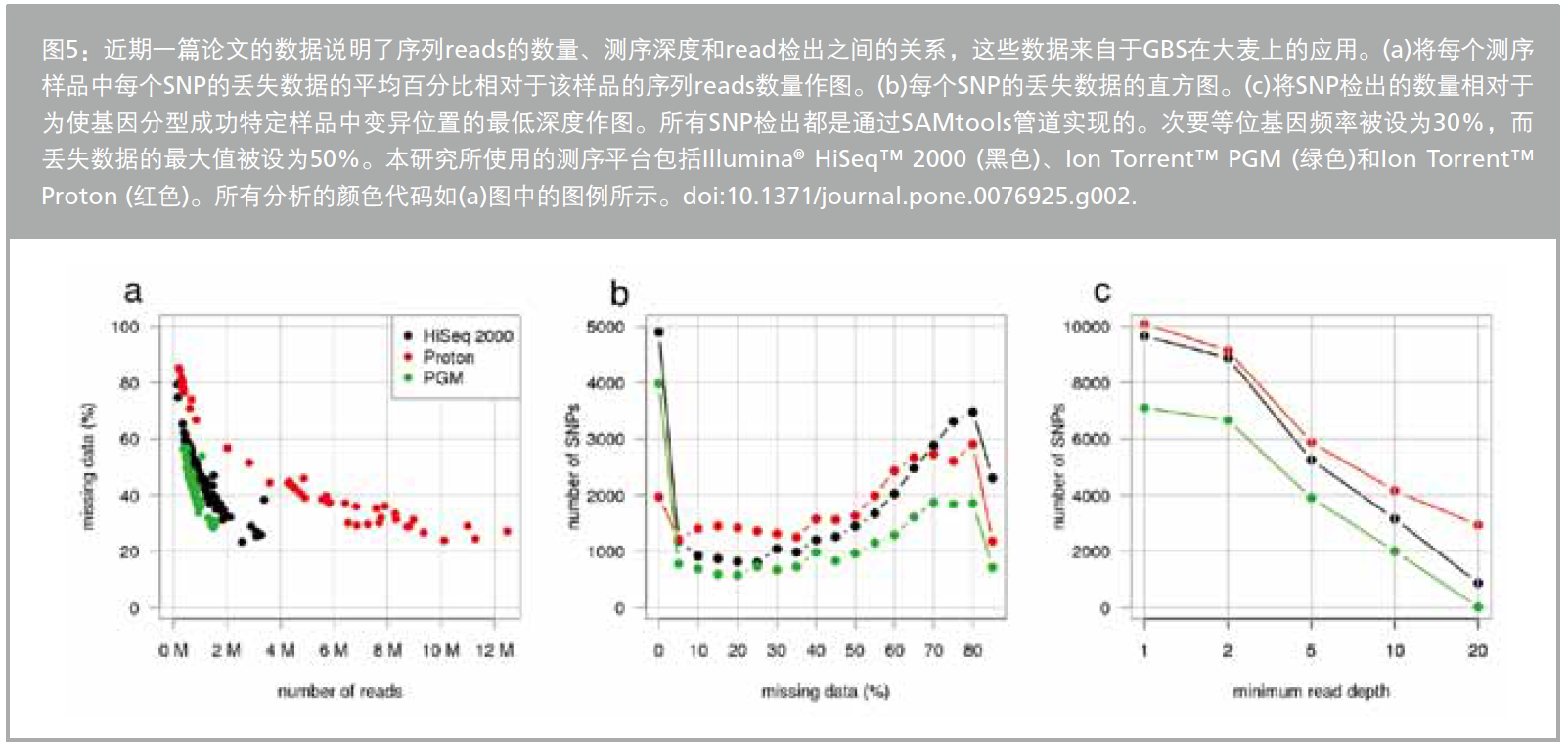
The expected and actual amounts of genotype data may vary greatly.A recent maize study using GBS18 showed that the oblique coverage and disproportionate regions of the genomic position distribution do not represent the initially expected information.This limits the scope and application of sequencing techniques and is considered to be unavailable for fine mapping of correlation studies.Genotype data at most loci can only be achieved by significantly increasing the read depth, which can affect sequencing costs.
Lost data can be recovered using data estimation technology, that is, comparing the data with the reference genome, which requires a lot of investment, advanced analysis, and complex processes that can filter, sort and compare sequence data.The lack of a simple and easy-to-use and unified informatics process remains the second biggest obstacle to the adoption of sequencing-based genotyping techniques in conventional applications.The calculation is particularly suitable for individuals with similar kinship, but for highly diverse samples, the lost data can be replaced with nearest neighbor alleles.19 When the proportion of lost data is high, sequencing-based genotyping techniques can also lose low-frequency alleles.The alternative is to pursue higher depth of sequencing, which leads to higher costs per sample.
LD and polymorphism frequency:For the population that collects genotype data, its genomic diversity and mating system have a great impact on sequencing costs.The population derived from a narrower genetic basis exhibits less polymorphism, requires more sequencing, and increases the overall cost.This is true for species such as tetraploid cotton, which exhibits a polymorphism every 1,000-1,500 bases.LD attenuation within species also determines the number of markers required for association mapping of multiple populations.Figure 6 shows the effect of LD attenuation on marker resolution.For species with high LD attenuation, low resolution of marker density will lead to insufficient coverage of the genome.Aquaculture species (such as trout) and plants (including corn, grapes, and beets) exhibit low LD, requiring a large number of fragments during correlation analysis.A recent genome-wide association study on trout20 used sequencing-based genotyping techniques and concluded that rapid attenuation of LD requires higher levels of marker density in order to efficiently conduct genome-wide association studies.
Copy Number Application:Chip and sequencing-based genotyping techniques can be used to conduct copy number studies to identify genetic variation in complex traits.Both techniques can be obtained by detecting copy numbers.However, sequencing-based genotyping techniques are difficult to identify copy number loss at low coverage because fragment loss is shown as a marker with low coverage.21 Higher coverage will enable detection of CNV loss, but the cost is expected to increase by 40-50%.
Genome complexity:Polyploidy is one of the more complex properties of plants and certain animals.60-70% of angiosperms are polyploid, and their ploidy levels range from tetraploidy in grape seeds to octaploidy in strawberries, while sugarcane is more complex, and their ploidy levels range from 12-16 times.Polyploid species exhibit genomic replication.The challenges of polyploid are as follows: (i) Polyploid species require higher sequence coverage to efficiently cover larger genomes, which increases sequencing costs.(ii) Genomic assembly and mapping algorithms are complex and error-prone, especially when assembling paralogic/orthologic regions.For polyploid and heterozygous species, data calculations for each specified site require complex analysis processes, which cannot be used in conventional breeding applications.22 In addition, deeper sequencing increases the total cost.Determining the allelic dose information at each locus in the genome is important for genome selection patterns.When genotyping polyploid species using chips, signals from the subgenome lead to cluster compression.Polyploid species also exhibit different levels of ploidy, as interfering mutations lead to reduced complexity.Conventional breeding applications must have an analysis process that automatically clusters and assigns genotypes to meet strict breeding time limit requirements.The Axiom® GT1 algorithm uses Bayesian statistics to accurately assign genotypes and cluster data from the polyploid genome.Figure 7 shows an example.Automatic processes allow people to easily and accurately genotypify thousands of markers from thousands of samples.
Guidelines for choosing the appropriate protocol for your genotyping project:Given advances in chip technology and sequencing technology, scientists need to recognize the challenges of using sequencing technology, as well as the bias of using sequencing and chip technology.The following questions can help you choose the appropriate technology to apply to the species considered in scientific research or genomic breeding projects:
n Are the analysis used to obtain genotype data compatible with the genomic structure of the species being considered and does it bring in sufficient amounts of reliable markers?
n Is there a need to target specific chromosomal regions and what marking strategies are needed to cover the entire genome?
n What is the potential LD structure of the species considered?
n Is this species polyploid and what is the level of ploidy?
n What kind of informatics processes and expertise is required to introduce this technology into a scientific research or breeding project?
n How many hours will it take to detect genotypes and cluster data?
n Are the groups considered inbred or diversified irrelevant individuals, and what is the expected level of heterozygousness?
n How many samples need to be genotyped, are there any restrictions on the turnover time or time to produce results?
n What depth of sequence coverage is required to accurately detect genotypes?
n What are the effects of data gaps and how will you recover the lost genotype?
n How cost of analysis considering the lost data and the resources required for bioinformatics processes and analysis?
n What is the flux, turnover time, analysis reliability and the instruments used by the technology?
n What is the amount of bias acceptable for a breeding program, and is there a way to bypass it?
n How many different techniques or analyses need to be integrated into scientific research or breeding projects for effective verification, marking traits or routine use?
Chip technology continues to develop to form the Axiom® 384HT format.This innovation that can process 384 samples at a very economical price point simultaneously has brought this technology from scientific research to mainstream commercial agricultural genomics.Chips remain the preferred technology in applications that prioritize turnover time, ease of use, and data quality.
Chip-based technology combines multiple genotyping applications on a single platform, providing flexibility and economicality.Innovations in analytical and informatics analysis processes allow all relevant markers of interest to be genotyping without restriction, and the results can be obtained in a few hours through a simple process.Axiom® Genotyping Solutions, Chip from Affymetrix
The technology evolution provides a complete solution for the application of genome-wide analysis to routine screening, with the highest accuracy and repetition, simplified processes and lowest costs.

The challenges of sequencing-based genotyping techniques are summarized in Table 2.
Table 2: Table 2 compares the characteristics of sequencing-based genotyping techniques (such as RADseq and GBS) with Axiom® Genotyping Arrays.New technologies are often promoted as reasons to replace chip technology because they ignore actual experimental conditions and genome complexity.

Affymetrix’s genotyping program for agricultural genomics provides breeders and researchers with a powerful and economical tool to identify, validate, and screen complex genetic traits in plants or animals for faster and more precise breeding.Axiom® genotyping starts by selecting marker content from the SNP library resources, then designing the SNP chip, and finally using the chip to identify the genotype of the sample.This provides breeders and researchers with a functional genotyping tool for application in marker-trait associations, genome-wide association studies (GWAS), quantitative trait loci (QTL) analysis and genome selection projects.
Given the challenges of genotyping-by-sequencing technology in data management and computing requirements, and the customized informatics process needs to be customized for each species and sample population, the chip is still in the application of data quality, integrity, analysis and conventional breeding.The easiest technology so far.
Overall, Axiom® genotyping solutions for animal and plant genotyping allow people to customize genotyping content on chips for commercially valuable species.Axiom genotyping solutions include species-specific and customized chips with proven genomic content from the Axiom® genome database, as well as complete kits, data analysis tools, and a fully automated utilizing GeneTitan® multi-channel (MC) instruments.process.
references and publications
1 Baird N. A., ETA. rapid SNP discovery and genetic mapping using sequence Dr AD markers. PL OS one 3:Oh 3376 (2008). do i:10.1371/journal.Broken.0003376
2 Elshire R. J., et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6:e19379 (2011). doi:10.1371/journal.pone.0019379
3 Narum S. R., et al. Genotyping-by-sequencing in ecological and conservation genomics. Molecular Ecology 22: 2841−2847 (2013). doi:10.1111/mec.12350
4 Peterson B. K., et al. Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 7(5):e37135 (2012).
do i:10.1371/journal.Broken.0037135
5 Kan ISA., ETA. development of high density 600K SNP genotyping array for chicken. BMC genomic S 14:59 (2013). do i:10.1186/1471-2164-14-59
6 Houston R. D., et al. Development and validation of a high density SNP genotyping array for Atlantic salmon (Salmo salar). BMC Genomics 15:90 (2014). doi:10.1186/1471-
2164-15-90
7 Affymetrix application note Mitigating sequencing errors, monomorphs, and poor performing markers during de novo SNP selection for genotyping applications (2013) P/N
DNA02261 Rev. 1
8 Ersoz E. S., Yu J., Buckler E. S. Applications of linkage disease and association mapping in crop plants, in Genomics-Assisted Crop Improvement: vol 1: Genomics
approaches and platforms, eds. var is NE R. K., tube Rosa R. springer, brand. 97-119 (2007). do i:10.1007/978-1-4020-6295-7_5
9 Poland J. A., et al. Genotyping-by-sequencing for plant breeding and genetics. The Plant Genome 5(3):92−102 (2012). doi:10.3835/plantgenome2012.05.0005
10 Meuwissen T., et al. Accelerating improvement of livestock with genomic selection. Annual Review of Animal Biosciences 1:221-237 (2013). doi:10.1146/annurevanimal-
031412−103705
11 Hui L., et al. An evaluation of genotyping by sequencing (GBS) to map the Breviaristatum-e (ari-e) locus in cultivated barley. BMC Genomics 15:104 (2014). doi:10.1186/1471-
2164-15-104
12 l IU S., ETA. development of the catfish 250K SNP array for genome-wide association studies. BMC research notes 7:135 (2014). do i:10.1186/1756-0500-7-135
13 Cavangh C. R., et al. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. 110(20):8057–8062.
do i:10.1073/Panas.1217133110
14 we were M. C., ETA. aspects of coverage in medical DNA sequencing. BMC bioinformatics 9:239 (2008). do i: 10.1186/1471-2105-9-239
15 HY MAK. E., G BS usage cases: non-model organisms. (2013). HTTP://大后大发. Job change. Cornell. Credit/Speaker/doc/GB S_non model_Sept_2013.PDF
16 Davey J. W., et al. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nature Reviews Genetics 12(7):499–510 (2011). doi:10.1038/
Beef Jerky 3012
17 Mascher M., et al. Application of genotyping-by-sequencing on semiconductor sequencing platforms: a comparison of genetic and reference-based marker ordering in barley.
PL OS one 8(10):Oh 76925 (2013). do i:10.1371/journal.0076925
18 Beissinger T. M., et al. Marker density and read depth for genotyping populations using genotyping-by-sequencing. Genetics 193(4):1073–1081 (2013). doi:10.1534/
genetics.112.147710
19 Huang X., et al. Genome-wide association studies of 14 agronomic traits in rice landraces. Nature Genetics 42(11):961−967 (2010). doi:10.1038/ng.695
20 Rexroad C. E., et al. Estimates of linkage disease and effective population size in rainbow trout. BMC Genetics 10:83 (2009). doi:10.1186/1471-2156-10-83
21 Donato MD et. al. Genotyping-by-sequencing (GBS): a novel, efficient and cost-effective genotyping method for cattle using next-generation sequencing. PLoS ONE 8(5):
Oh 62137. do i:10.1371/journal.Broken.0062137.
22 B rummer, ETA. applied genetics and genomic sin alfalfa breeding. agronomy 2:40-61 (2012). do i:10.3390/agronomy2010040
Agrogenomics solutions from Affymetrix provide breeders and researchers with a powerful and flexible genotyping tool,
It can cost-effectively identify, verify and screen complex genetic traits in plants and animals.
Agrogenomics solutions from Affymetrix provide breeders and researchers with a powerful and flexible genotyping tool,
It can cost-effectively identify, verify and screen complex genetic traits in plants and animals.
Agricultural Genomics Solutions from Affymetrix