主要功能
本系统是全球检出限和灵敏度很高的乙烯监测系统,主要用于植物研究相关的乙烯气体监测,如种子发芽、植物生长发育、开花生理、植物器官衰老、基因表达、植物病原体相互作用、植物激素间相互作用、蔬果收货后保藏、植物抗逆性研究(干旱、高温、重金属)等。
其中乙烯气体检测仪 ETD-300 采用先进的激光技术(光声学原理),即样品乙烯在光声腔吸收激光后释放热使光声腔内部产生压力,随激光频率增减形成能被微型麦克风检测到的压力差,而乙烯浓度越高压力差越大,从而据声波强度差可实时快速测量乙烯气体(C2H4)绝对浓度;阀门控制箱 VC-6 完全自动化和电脑控制,接一个即可以使单个气体检测仪实现6个样品的自动切换测量,单个乙烯气体检测仪可以接一个或多个阀门控制箱;烃分解器 CAT-1 则利用铂金颗粒催化烃氧化分解为水蒸气和 CO2,为系统提供无烃干扰的样品空气。
测量参数
测量参数:乙烯浓度(ppbv)、气体流速(l/h)、背景值、模拟输入(V)
计算参数:乙烯产量(nl/h)
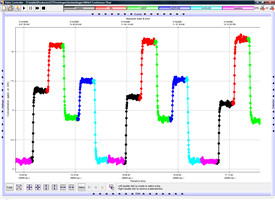
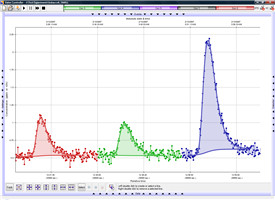
连续流动测定(左)和积累测定(右)的乙烯监测数据图
应用领域
用于环境、医学、农业、工业、生态、生物等监测领域。特别适合植物生理、发育研究的超灵敏乙烯测量。



主要技术参数
参数 | 乙烯气体检测仪 ETD-300 | 阀门控制箱 VC-6 | 烃分解器 CAT-1 | |
测量范围 | 0-2 ppm / 0-100 ppm(可调) | / | / | |
检出限 | 0.3 ppbv | / | / | |
噪音(2σ) | 0.3 ppbv | / | / | |
精度 | <1% 或 0.3 ppbv | 0.2% FS | / | |
稳定性 | <1% 超过 24 小时 | / | / | |
零点漂移 | +/-1 ppbv | / | / | |
测量时间 | 7-9 s | / | / | |
响应时间 | 30 s (当流量为1 l/h时) | 300 ms | / | |
流量 | 0.25-5 l/h | 0.25-5 l/h | 0-30 l/h | |
校准 | 使用标准混合气,每年一次 | / | / | |
通道数量 | / | 6(可增至 12, 18 等) | / | |
测量模式 | / | 连续测量,积累测量 | / | |
气体供应压力 | / | 0.5-5 Bar | / | |
过压阀 | / | 在 5 Bar 时打开 | / | |
滤膜类型 | / | 去除粒径 >7μm 的微粒 | / | |
最大稀释浓度 | / | / | 100 ppm | |
输出浓度 | / | / | < 100 pptv | |
压力 | / | / | 0-6 atm | |
活性催化剂 | / | / | Pt/SiO2 | |
催化温度 | / | / | 150–250 ℃ | |
预热时间 | 30 min | / | < 10 min | |
尺寸 | 42x45x14 cm (48.3cm 3U 机架) | 30x45x10 cm (48.3cm 2U机架) | 33x24x14 cm (48.3 cm 3U 半机架) | |
工作温度/湿度 | 10-28 ℃ / 0-95 % RH | 5-40 ℃ / 0-95 % RH | 5-40 ℃ / 0-95 % RH | |
电源要求 | 90-264 VAC,47-63 Hz | 90-264 VAC,47-63 Hz | 90-264 VAC,47-63 Hz | |
功耗 | <150 W | <20 W | 85 W | |
进气接口 | 接外径 1/8'' 软管的快速接头 | 接外径 1/8'' 软管的快速接头 | 接外径 1/8'' 软管的快速接头 | |
模拟输入 | 0-5 V | / | / | |
数据输出 | USB,CSV 格式 | USB,CSV 格式 | / | |
显示 | 触摸屏 | LED 指示灯 | / |
选购指南:
6通道监测系统组成如下:



乙烯气体检测仪ETD-300 + 阀门控制箱VC-6 + 烃分解器CAT-1
注:系统中 3 个仪器都可以单独使用
可酌情选择单通道系统:乙烯气体检测仪 ETD-300+ 烃分解器 CAT-1。
产地:荷兰Sensor Sense 
应用举例
1.1 乙烯测定在高温胁迫研究中的应用举例
实验内容简介:以生长 3 周的拟南芥野生型 Col-0,突变体 NahG 和 opr3 植株为材料,研究了其高温胁迫下的乙烯释放。其中,野生型 Col-0 高温胁迫(38℃)下,电导率(电解质渗透率)、水杨酸和茉莉酸含量和乙烯释放增加;突变体 NahG 和 opr3 高温胁迫(38℃)下电导率、茉莉酸和乙烯释放也增加,但都低于野生型 Col-0,而高温胁迫后恢复阶段(水中 22℃)电导率明显高于 Col-0。研究结果表明:高温胁迫下,乙烯迅速产生,其生产受到茉莉酸和水杨酸的调控。总的来说,茉莉酸与水杨酸协同调节植物对高温胁迫的耐受,而乙烯主要加快细胞死亡;突变体 NahG 和 opr3 比野生型 Col-0 的耐热性差,细胞死亡多。
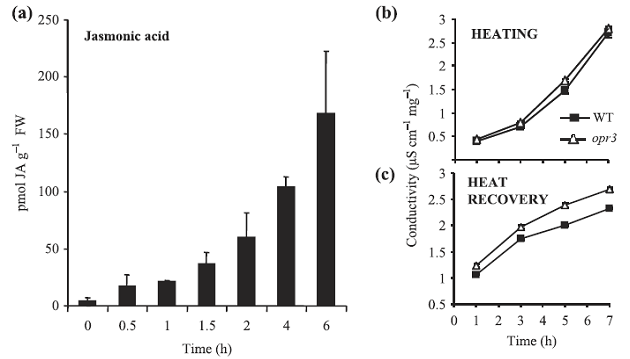
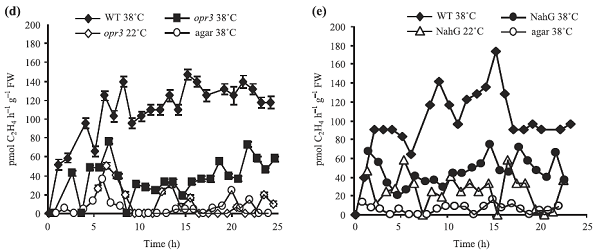
图1 高温处理下拟南芥植株的水杨酸(a)、电导率(b、c)和乙烯释放(d、e)
WT:拟南芥野生型;突变株opr3 ;突变株NahG以及培养基agar
Clarke, S.M., et al., Jasmonates act with salicyli c acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytologist, 2009. 182(1): p. 175-187.
1.2 乙烯测定在营养缺乏(Mg)胁迫研究中的应用举例
实验内容简介:以生长5周的水培拟南芥 Col-0 植株为材料,研究了其缺镁胁迫下的乙烯释放。缺镁处理后乙烯生物合成酶基因(例如 At5g43450、At1g06620 和At2g25450)的表达水平明显上升,样品乙烯释放是对照组的两倍多,叶片中抗坏血酸 ASC 和谷胱甘肽 GSH 的氧化态比例增加。研究结果表明:植物应答缺镁胁迫存在一些独特的信号通路,且与植物激素有关,而乙烯在应答缺镁过程中发挥了关键作用;缺镁还同步增强了植物抗氧化酶活性。
表 1 镁元素缺乏处理第 8 天拟南芥新成熟叶片和根系的生理参数

DHA:ASC,氧化态脱氢抗坏血酸:抗坏血酸;GSSG : GSH,氧化型谷胱甘肽:谷胱甘肽;Ctrl,镁元素充足的植株;-Mg,镁元素缺乏的植株
Hermans, C., et al., Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytologist, 2010. 187(1): p. 132-144.
1.3 乙烯测定在病菌感染研究中的应用举例
实验内容简介:以品种为 Money Maker 和 Daniela 的成熟番茄果实为材料,研究了其感染番茄灰霉病菌株 VTF1 的乙烯释放。灰霉病菌可以在体外产生乙烯,其乙烯释放与其说与分生孢子萌发相关,不如说与菌丝生长更相关,且分生孢子浓度越大真菌的乙烯释放越多。感染灰霉病的两种番茄的乙烯释放规律与灰霉病菌类似;但释放量是其 100 倍。结合受感染番茄的细胞学参数,研究结果表明:番茄-真菌系统的乙烯释放不是由番茄灰霉病菌引起的,虽说与其内部的真菌生长速率十分同步。
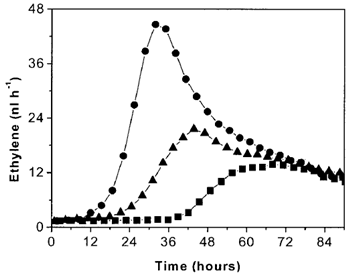
图 2 真菌(160 μl 悬浮液)的乙烯产量
● 1.5*108 灰霉病菌分生孢子 ml-1 ▲ 2*107 灰霉病菌分生孢子 ml-1 ■ 2*105 灰霉病菌分生孢子 ml-1
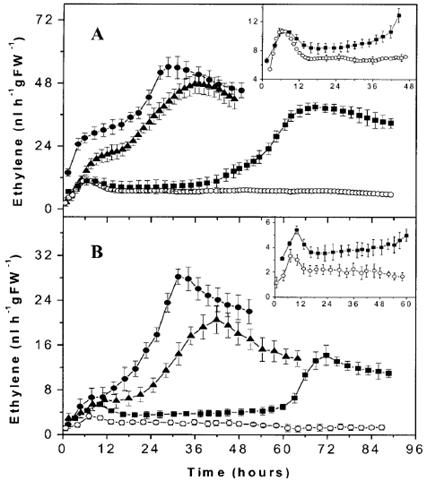
图3 模拟感染和不同浓度番茄灰霉病菌感染的两种番茄的乙烯释放
A.番茄品种 Money Maker;B.番茄品种 Daniela;
○ 模拟番茄灰霉病菌感染 ● 1.5*108 灰霉病菌分生孢子 ml-1 ▲ 2*107 灰霉病菌分生孢子 ml-1 ■ 2*105 灰霉病菌分生孢子 ml-1
Cristescu, S.M., et al., Ethylene Production by Botrytis cinerea In Vitro and in Tomatoes. Applied and Environmental Microbiology, 2002. 68 (11): p. 5342-5350.
参考文献
原始数据来源:Google Scholar
M. Anastasiadi, et al. (2016). "Tissue biochemical diversity of 20 gooseberry cultivars and the effect of ethylene supplementation on postharvest life." Postharvest Biology and Technology 117: 141-151.
M. M. A. Bisson, et al. (2016). "Peptides interfering with protein-protein interactions in the ethylene signaling pathway delay tomato fruit ripening." Scientific Reports 6: 30634.
I. Bulens, et al. (2014). "Dynamic changes of the ethylene biosynthesis in ‘Jonagold’ apple." Physiologia Plantarum 150(2): 161-173.
N. Busatto, et al. (2016). "Candidate gene expression profiling reveals a time specific activation among different harvesting dates in ‘Golden Delicious’ and ‘Fuji’ apple cultivars." Euphytica 208(2): 401-413.
R. Centeno, et al. (2014). "Three mirror off axis integrated cavity output spectroscopy for the detection of ethylene using a quantum cascade laser." Sensors and Actuators B: Chemical 203: 311-319.
J. Chmielewska-Bak, et al. (2014). "Effect of cobalt chloride on soybean seedlings subjected to cadmium stress." Acta Societatis Botanicorum Poloniae 83(3).
S. M. Cristescu, et al. (2015). Research Tools: Ethylene Detection.C.-K. Wen. Dordrecht, Springer Netherlands: 263-286.
T. Dawood, et al. (2016). "A Co-Opted Hormonal Cascade Activates Dormant Adventitious Root Primordia upon Flooding in Solanum dulcamara." Plant Physiology.
H. De Gernier, et al. (2016). "A Comparative Study of Ethylene Emanation upon Nitrogen Deficiency in Natural Accessions of Arabidopsis thaliana." Frontiers in Plant Science 7: 70.
E. Gharbi, et al. (2016). "Salicylic acid differently impacts ethylene and polyamine synthesis in the glycophyte Solanum lycopersicum and the wild-related halophyte Solanum chilense exposed to mild salt stress." Physiologia plantarum 158(2): 152-167.
S. W. Hoogstrate, et al. (2014). "Tomato ACS4 is necessary for timely start of and progression through the climacteric phase of fruit ripening." Frontiers in Plant Science 5: 466.
A. Jabbar and A. R. East (2016). "Quantifying the ethylene induced softening and low temperature breakdown of ‘Hayward’ kiwifruit in storage." Postharvest Biology and Technology 113: 87-94.
M. Keshavarzi, et al. (2014). "Ethephon and secondary shoot induction in Gentian (Gentiana spp.) hybrids in vitro." Scientia Horticulturae 179: 170-173.
Martin Schäfer, et al. (2015). "Cytokinin concentrations and CHASE-DOMAIN CONTAINING HIS KINASE 2 (NaCHK2)- and NaCHK3-mediated perception modulate herbivory-induced defense signaling and defenses in Nicotiana attenuata." The New phytologist 207(3): 645-658.
N. A. Mohd-Radzman, et al. (2016). "Different pathways act downstream of the peptide receptor CRA2 to regulate lateral root and nodule development." Plant Physiology.
D. Nguyen, et al. (2016). "Drought and flooding have distinct effects on herbivore-induced responses and resistance in Solanum dulcamara." Plant, Cell & Environment 39(7): 1485-1499.
K. Razzaq, et al. (2015). "Role of 1-MCP in regulating 'Kensington Pride' mango fruit softening and ripening." Plant Growth Regulation: 1-11.
S. Rupavatharam, et al. (2015). "Re-evaluation of harvest timing in ‘Unique’ feijoa using 1-MCP and exogenous ethylene treatments." Postharvest Biology and Technology 99: 152-159.
S. Rupavatharam, et al. (2016). "Effects of preharvest application of aminoethoxyvinylglycine (AVG) on harvest maturity and storage life of ‘Unique’ feijoa." New Zealand Journal of Crop and Horticultural Science 44(2): 121-135.
R. Santhanam, et al. (2014). "Analysis of Plant-Bacteria Interactions in Their Native Habitat: Bacterial Communities Associated with Wild Tobacco Are Independent of Endogenous Jasmonic Acid Levels and Developmental Stages." PLoS ONE 9(4): e94710.
K. Schellingen, et al. (2014). "Cadmium-induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression." BMC Plant Biology 14(1): 1-14.
A. Sivakumaran, et al. (2016). "ABA Suppresses Botrytis cinerea Elicited NO Production in Tomato to Influence H(2)O(2) Generation and Increase Host Susceptibility." Frontiers in Plant Science 7: 709.
R. Valluru, et al. (2016). "Foliar Abscisic Acid-To-Ethylene Accumulation and Response Regulate Shoot Growth Sensitivity to Mild Drought in Wheat." Frontiers in Plant Science 7: 461.
B. Van de Poel, et al. (2016). "Transcriptome Profiling of the Green Alga Spirogyra pratensis (Charophyta) Suggests an Ancestral Role for Ethylene in Cell Wall Metabolism, Photosynthesis, and Abiotic Stress Responses." Plant Physiology 172(1): 533-545.
D. Vromman, et al. (2016). "Salinity influences arsenic resistance in the xerohalophyte Atriplex atacamensis Phil." Environmental and Experimental Botany 126: 32-43.
R. L. Wilson, et al. (2014). "Loss of the ETR1 ethylene receptor reduces the inhibitory effect of far-red light and darkness on seed germination of Arabidopsis thaliana." Frontiers in Plant Science 5: Article 433(431-413).
R. L. Wilson, et al. (2014). "The Ethylene Receptors ETHYLENE RESPONSE1 and ETHYLENE RESPONSE2 Have Contrasting Roles in Seed Germination of Arabidopsis during Salt Stress." Plant Physiology 165(1532-2548 (Electronic)): 1353–1366.
A. Xu, et al. (2014). "ENHANCING CTR1-10 ETHYLENE RESPONSE2 is a novel allele involved in CONSTITUTIVE TRIPLE-RESPONSE1-mediated ethylene receptor signaling in Arabidopsis." BMC Plant Biology 14: 48-48.
Z. S. Zahoor Hussain (2015). "Involvement of ethylene in causation of creasing in sweet orange [Citrus sinensis (L.) Osbeck] fruit." Australian Journal of Crop Science 9(1): 1-8.
