Main functions
Using on-board chip LED array technology, 6 different bands of excitation light are used as measurement light, actinic light, saturation pulse, single turnover saturation flash and multi turnover saturation flash
It has 200 times more sensitive than PAM-2500
Can be used for measurement of very dilute suspensions (algae, chloroplast suspension)
Special leaf clips can be used for measurement of leaf-like samples such as higher plants/large seaweed
Standard PAM measurement function, complex multiphase fluorescence ascending kinetic fit analysis, relaxation kinetic analysis
Especially suitable for state transition research, "Inactive PSII" ("Inactive PSII") research
Ultrafast time resolution reaches 10 ms, thus using a unique O-I1 phase (O-J phase) fitting analysis was used to analyze the central heterogeneity analysis of PSII reflecting, resulting in connectivity parameters (p and J) of PSII photosynthetic units.), rate constant (Tau) and optical cross-sectional area of two different types of PS II (Type 1 and Type 2) (Sigma(II)λ) and other parameters
Added PSII effective light intensity PAR(II), absolute electron transfer rate ETR(II) through PSIIλetc. new photosynthetic parameters.
Supporting operation software for complex fitting analysis
Measurement parameters
F Oh, FM, F, FM, F V/FM, Y(II), QP, QN, NP Q, Y(no), Y(NP Q), ET R, ET R(II)λ, fear, J, τ, sigma(II)λ, PAR, PAR(II) etc.
Application areas
It is mainly used for in-depth photosynthesis mechanism research of various algae, and in-depth research on cyanobacteria, green algae, diatoms, dinoflagellates, red algae, cryptoalgae, etc. with suitable wavelengths, new measurements, and new parameters.If you choose higher plant attachments, you can also measure the leaves of higher plant.
Main technical parameters
Measured light: Provides pulse-modulated measurement light of 400, 440, 480, 540, 590 and 625 nm, 20 intensity selections, 14 frequency selections.
Actinic light: Provide continuous actinic light from 440, 480, 540, 590, 625 nm and 420-640 nm (white light), with maximum light intensity of 4000 μmol m-2yes-1;Maximum intensity of single turnover saturated flash 200 000 μmol m-2yes-1, duration 5-50 μs adjustable; multi-turnover saturated flash intensity 10 000 μmol m-2yes-1, adjustable from 1-800 ms.
Far red light: 725 nm.
Signal detection: PIN-photodiode with special phase locked amplifier (patented design), maximum time resolution of 10 μs.
Introduction to the functions of Multi-Color-PAM
The relative electron transfer rate rETR of optical system II is a very commonly used parameter.rETR = PAR × Y(II) × ETR-factor, where ETR-factor refers to the proportion of light energy absorbed by the optical system II to the total incident PAR.In most published literatures, no attempt was made to determine ETR-factor, but simply assumed that it was the same as "mode blades", that is, 50% of the PAR was distributed to the optical system II and 84% of the PAR was absorbed by photosynthetic pigments.Therefore, in existing literature, rETR is generally calculated using the formula rETR = PAR × Y(II) × 0.84 × 0.5.
Recently, the absolute electron transfer rate of optical system II can be achieved by using multi-excitation wavelength modulated chlorophyll fluorescence meter MULTI-COLOR-PAMλmeasurement.First, use MULTI-COLOR-PAM to determine the functional optical cross-sectional area of optical system II at a certain wavelength Sigma(II)λ(Unit nm2) (where λ is the wavelength), then find the quantum absorption rate of optical system II PAR(II) = Sigma(II)λ× L × PAR = 0.6022 × Sigma(II)λ× PAR.where L is the Avogadro constant and the coefficient 0.6022 is to 1 μmol quanta m-2(i.e. 6.022 × 1017 quanta m-2) Convert to 0.6022 quanta nm-2, the unit of PAR(II) is quanta/(PSII × s).Next, you can calculate ETR(II)λ= PAR(II) × Y(II)/Y(II)max, where Y(II)max is the quantum yield of the optical system II after dark adaptation, that is, Fv/Fm×ETR(II)The unit is electrons/(PSII × s).
Traditional modulated chlorophyll fluorescence instruments generally can only provide one or two colors of light sources, such as halogen lamps that emit white light, blue LEDs that emit blue light, or red LEDs that emit red light, etc.The results of measuring light with different colors may vary. As shown in Figure 1A, there is a very significant difference in the rapid light curve of the green algae Chlorella Chlorella illuminated by blue light (440 nm) and red light (625 nm).The rETRmax under the irradiation is significantly smaller than that under the red light irradiation, and there is a slight downward trend in the stronger light curve rETR, which shows that the blue light is more likely to induce light suppression (Schreiber, Klughammer et al. 2011, Schreiber, Klughammer et al. 2012).From this, it can be inferred that many experimental results reported in the past literature may have misunderstandings caused by different excitation light sources used.
As mentioned above, using MULTI-COLOR-PAM, the real electron transfer rate ETR(II) can already be measuredλ.If using ETR(II)λWhat will happen if you draw a fast light curve?Figure 1B is the result obtained after converting the results of Figure 1A into absolute electron transfer rate. It can be seen that the absolute electron transfer rate is consistent whether it is irradiated with blue light or red light.This proves that the difference in the results in Figure 1A is due to the optical system II functional optical cross-sectional area of algae cells at different wavelengths Sigma(II)λdifferent sizes of the , (Schreiber, Klughammer et al. 2011, Schreiber, Klughammer et al. 2012).This utilizes absolute electron transfer rate ETR(II)λThe drawn fast light curves may play an increasingly important role in future scientific research.
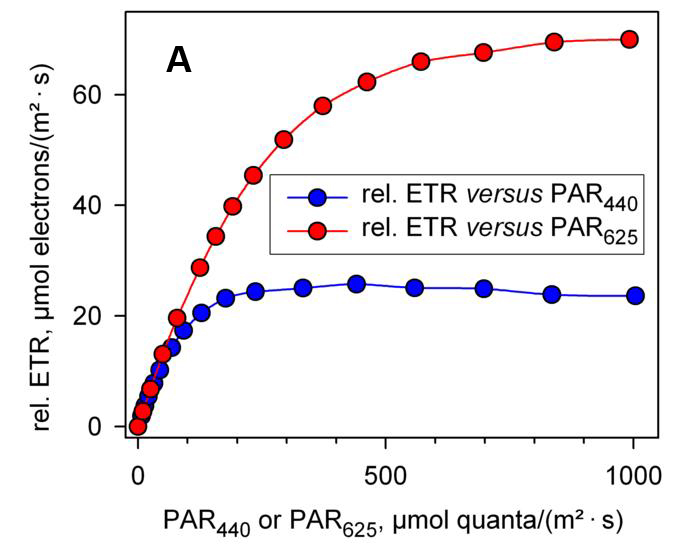 | 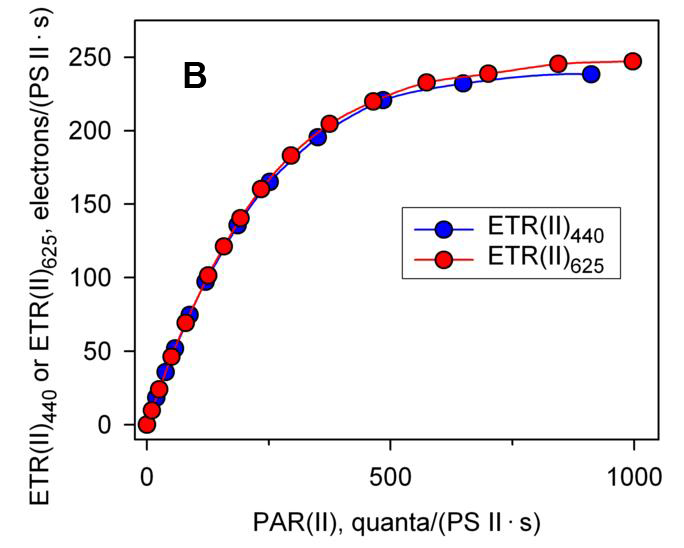 |
| Figure 1 Fast light curves drawn using relative electron transfer rate (A) and absolute electron transfer rate (B) respectively (cited from Schreiber et al., 2012) | |
| The rapid light curve of Chlorella sp. was measured using MULTI-COLOR-PAM with blue light (440 nm) and red light (625 nm) as actinic light sources, respectively. | |
| In Figure A, rETR calculation uses 0.42 as the ETR factor. | |
| In Figure B, the functional optical cross-sectional area of the optical system II obtained under the excitation of blue and red light Sigma(II)λ4.547 and 1.669 nm respectively2, calculate the absolute electron transfer rate ETR(II)440and ETR(II)625The Fv/Fm of the two groups are 0.68 and 0.66, respectively. | |
Purchase Guide
1. Basic hanging sample measurement
System composition: general-purpose host, standard version detection unit, optical unit of suspension, data cable, workbench, software, etc.
 |
| Basic hanging sample measurement model |
2. Basic articles on measuring leaves of higher plants
System composition: general-purpose host, standard version detection unit, special blade clip, data cable, workbench, software, etc.
 |
| Special leaf clips for measuring leaves of higher plants |
3. Other optional accessories
1. ED-101US/T: The temperature control device, installed on ED-101US/MD, is a suspension liquid temperature control; it can be connected to an external circulating water bath to control the temperature,
2. US-SQS/WB: Spherical miniature optical quantum probe, which can be inserted into the sample cup to measure PAR; controlled by the host DUAL-C.
3. PHYTO-MS: Magnetic stirrer, connected to the bottom of the optical unit ED-101US/MD to stir the suspension.
Origin: WALZ, Germany
References
Data source: Photosynthesis Literature Endnote database, updated to January 2021, with more than 10,000 documents
Original data source: Google Scholar
1.Grund, M., et al. (2022). "Heterologous Lactate Synthesis in Synchocystis sp. Strain PCC 6803 Causes a Growth Condition-Dependent Carbon Sink Effect." Applied and Environmental Microbiology 0(0): e00063-00022.
2.Xiao, X., et al. (2022). "Effects of three graphene-based materials on the growth and photosynthesis of Brassica napus L." Ecotoxicology and Environmental Safety 234: 113383.
3.Xie, S., et al. (2022). "Enhanced lipid productivity coupled with carbon and nitrogen removal of the diagnostic Skeletonema costatum cultured in the high CO2 level." Algal Research 61: 102589.
4.Bernát, G., et al. (2021). "Photomorphogenesis in the Picocyanobacterium Cyanobium gracile Includes Increased Phycobilisome Abundance Under Blue Light, Phycobilisome Decoupling Under Near Far-Red Light, and Wavelength-Specific Photoprotective Strategies." Frontiers in Plant Science 12(352).
5.Ma, Z., et al. (2021). "Inhibitory effects of Prorocentrum donghaiense allelochemicals on Sargassum fusiformis zygotes probed by JIP-test based on fast chlorophyll fluoresce kinetics." Marine environmental research 170: 105453.
6.MATTILA, H., et al. (2021). "Differences in susceptibility to photoinhibition do not determine growth rate under modernate light in batch or turbidostat–a study with five green algae." Photosynthetica.
7.Mehmood, S. S., et al. (2021). "Integrated analysis of transcriptomics and proteomics provide insights into the molecular regulation of cold response in Brassica napus." Environmental and Experimental Botany 187: 104480.
8.Michel-Rodriguez, M., et al. (2021). "Underwater light climate and wavelength dependence of microalgae photosynthesis parameters in a temperature sea." PeerJ 9: e12101.
9.Qu, L., et al. (2021). "Elevated pCO2 enhances under light but reduces in darkness the growth rate of a diagnosis, with implications for the fate of physitoplankton below the photo zone." n/a(n/a).
10.Schansker, G. (2021). "Kinetic characterization of the interaction of NO with the S2 and S3 states of the oxygen-evolving complex of Photosystem II." bioRxiv: 2021.2001.2010.426130.
11.Schreiber, U. and C. K passes by hammer (2021). "evidence for variable chlorophyll fluorescience of photo system II n vivo." photosynthesis research.
12.Sukačová, K., et al. (2021). "Perspective Design of Algae Photobioreactor for Greenhouses—A Comparative Study." energies 14(5): 1338.
13.Terentyev, V. V. (2021). "Loss of carbonic anhydrase in the thylakoid lumen causes unusual moderate-light-induced rearrangement of the chloroplast in Chlamydomonas reinhardtii as a way of photosystem II photoprotection." Plant Physiology and Biochemistry 168: 501-506.
14.Wang, C., et al. (2021). "Harmful algal bloom-forming dinoflagellate Prorocentrum donghaiense inhibits the growth and photosynthesis of seaweed Sargassum fusiformis embryos." Journal of Oceanology
15.limnology: 1-15.
16.Zavafer, A., et al. (2021). "Normalized chlorophyll fluorescience imaging: A method to determine irradiance and photosynthetically active radiation in physitoplankton cultures." Algal Research 56: 102309.
17.Zavřel, T., et al. (2021). "Monitoring fitness and productivity in cyanobacteria batch cultures." Algal Research 56: 102328.
18.Andrzejczak, O. A., et al. (2020). "The Hypoxic Proteome and Metabolome of Barley (Hordeum vulgare L.) with and without Phytoglobin Priming. ." Int. J. Mol. Sci(21): 1546.
19.Chen, Y., et al. (2020). "Astaxanthin biosystemthesis in transgenic Dunaliella salina (Chlorophyceae) enhanced tolerance to high irradiation stress." South African Journal of Botany 133: 132-138.
20.P av aux, A.-S. (2020). "chemical ecology of the toxic low no flaagellate OST Re op sis teahouse. OVA inn.W. Mediterranean sea."Dissertation.
twenty one.S touched OVA, T., ETA. (2020). "cortical photosynthesis ASA physical marker for grape breeding: methods and approaches." bio Web of conferences 25: 02018.
twenty two.Terentyev, V. V., et al. (2020). "The Main Structural and Functional Characteristics of Photosystem-II-Enriched Membranes Isolated from Wild Type and cia3 Mutant Chlamydomonas reinhardtii." Life(10): 63.
twenty three.Zhang, X., ETA. (2020). "Photosynchronthetic properties of MIS can thus con Sat volcanically devastated site son MI Dental-Gima Island." plants(9): 1212.
twenty four.Grund, M., et al. (2019). "Electron balancing under different sink conditions reveals positive effects on photon efficiency and metabolic activity of Synechocystis sp. PCC 6803." Biotechnology for Biofuels 12(1): 43.
25.Røkke, G. B., et al. (2019). "Unique photosynthesis electron transport tuning and excitation distribution in heterokont algae." PLoS ONE 14(1): e0209920.
26.Schreiber, U., et al. (2019). "Rapidly reversible chlorophyll fluorescience quenching induced by pulses of supersaturating light in vivo." Photosynthesis Research: 1-16.
27.Wang, W. and Y. Sheng (2019). "Pseudomonas sp. strain WJ04 enhances current generation of Synechocystis sp. PCC6803 in photomicrobial fuel cells." Algal Research 40: 101490.
28.Yanykin, D., et al. (2019). "Hydroxyectoine protects Mn-depleted photosystem II against photoinhibition acting as a source of electrons." Photosynthesis Research: 1-15.
29.Chartrand, K. M., et al. (2018). "Living at the margins–The response of deep-water seagrasses to light and temperature renders them susceptible to acute impacts." Marine environmental research.
30.Goessling, J. W., et al. (2018). "Modulation of the light field related to valve optical properties of raphid diagnosis: implications for niche differentiation in the microphytobenthos." MARINE ECOLOGY PROGRESS SERIES 588: 29-42.
31.Khorobrykh, A., et al. (2018). "Photooxidation and photoreduction of exogenous cytochrome c by photosystem II preparations after various modifications of the water-oxidizing complex." Photosynthetica: 1-10.
32.Li, F., et al. (2018). "Diatom performance in a future ocean: interactions between nitrogen limitation, temperature, and CO2-induced seawater awareness." ICES Journal of Marine Science.
33.Lin, L., et al. (2018). "Electrochemical oxidation of Microcystis aeruginosa using a Ti/RuO2 anode: contributions of electrochemically generated chlorines and hydrogen peroxide." Environmental Science Pollution Research.
34.Miao, H., et al. (2018). "Calcification Moderates the Increased Susceptibility to UV Radiation of the Coccolithophorid Gephryocapsa oceanica Grown under Elevated CO 2 Concentration: Evidence Based on Calcified and Non‐calcified cells." photochemistry stabilizes photobiology.
35.Morelle, J. and P. Claquin (2018). "Electron requirements for carbon incorporation along a diel light cycle in three marine diagnostic species." Photosynthesis Research 137(2): 201-214.
36.Morelle, J., et al. (2018). "Annual Phytoplankton Primary Production Estimation in a Temperate Estuary by Coupling PAM and Carbon Incorporation Methods." Estuaries and Coasts: 1-19.
37.NI Look at um, l., ETA. (2018). "regulation of chloroplast NAD H dehydrogenase-like complex by NAD pH-dependent TH IO red o-mind system." Bior Xi V: 261560.
38.Preuss, M. and G. C. Zuccarello (2018). "Comparative studies of photosynthesis capacity in three pigmented red algal parasites: Chlorophyll a concentrations and PAM fluorometry measurements." Phycological Research 0(0).
39.sung, M.-G., ETA. (2018). "wavelength shift strategy to enhance lipid productivity of NaN no or op sis GA carpet A." biotechnology for biofuels 11(1): 70.
40.Ternon, E., et al. (2018). "Allelopathic interactions between the benthic toxic dinoflagellate Ostreopsis cf. ovata and a co-occurring diagnosis." Harmful Algae 75: 35-44.
41.Zavřel, T., et al. (2018). "Effect of carbon limitation on photosynthesis electron transport in Nannochloropsis oculata." Journal of Photochemistry and Photobiology B: Biology 181: 31-43
42.Béchet, Q., et al. (2017). "Modeling the impact of high temperatures on microalgal viability and photosynthesis activity." Biotechnology for Biofuels 10(1): 136.
43.Havurinne, V. and E. Tyystjärvi (2017). "Action spectrum of photoinhibition in the diagnosis Phaeodactylum tricornutum." Plant and Cell Physiology: pcx156.
44.KA Garbage, M. H., ETA. (2017). chlorophyll fluorescence: understanding crop performance—basics and applications, CRC press.
45.Lamb, J. J. and M. F. Hohmann-Marriott (2017). "Manganese acquisition is facilitated by PilA in the cyanobacterium Synechocystis sp. PCC 6803." PLoS ONE 12(10): e0184685.
46.Røkke, G., et al. (2017). "The plastoquinone pool of Nannochloropsis oceanica is not completely reduced during bright light pulses." PLoS ONE 12(4): e0175184.
47.sav Chen Kun Oh, T., ETA. (2017). "the hydro peroxide l Arthur branch of Theo XY gift pathway protects against photo inhibition of photosynthesis." plant A: 1-14.
48.Shin, W.-S., et al. (2017). "Complementation of a mutation in CpSRP43 causing partial truncation of light-harvesting chloride antenna in Chlorella vulgaris." Scientific Reports 7(1): 17929.
49.Laviale, M., et al. (2016). "The importance of being fast: comparative kinetics of vertical migration and non-photochemical quenching of benthic diagnosis under light stress." Marine Biology 163(1): 1-12.
50.Murphy, T. E., et al. (2016). "A radical transfer modeling approach for accurate interpretation of PAM fluorometry experiments in suspended algal cultures." Biotechnology Progress: n/a-n/a.
51.Shin, W.-S., et al. (2016). "Truncated light-harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity." Journal of Applied Phycology: 1-10.
52.Yanykin, D. V., et al. (2016). "Trehalose protects Mn-depleted photosystem 2 preparations against the donor-side photoinhibition." Journal of Photochemistry and Photobiology B: Biology 164: 236-243.
53.He, J., et al. (2015). "Photoinactivation of Photosystem II in wild-type and chloride b-less barley leaves: which mechanism dominates depends on experimental circumstances." Photosynthesis Research: 1-9.
54.Lin, L., et al. (2015). "Effects of electrolysis by low-amperage electric current on the chlorophyll fluorescience characteristics of Microcystis aeruginosa." Environmental Science and Pollution Research: 1-8.
55.Polishchuk, A., et al. (2015). "Cultivation of Nannochloropsis for eicosapentaenoic acid production in wastewaters of pulp and paper industry." Bioresource Technology 193: 469-476.
56.They are not daily, B., ETA anymore. (2015). "gas transfer controls carbon limitation during biomass production by Marine microalgae." Chem SU Chem.
57.Yanykin, D., et al. (2015). "Trehalose stimulation of photoinduced electron transfer and oxygen photoconsumption in Mn-depleted photosystem 2 membrane fragments." Journal of Photochemistry and Photobiology B: Biology 152: 279-285.
58.Klughammer, C. and U. Schreiber (2014). Apparent PS II absorption cross-section and estimation of mean PAR in obviously thin and dense suspensions of Chlorella. Photosynth Res.
59.Szabó, M., K. Parker, et al. (2014). Photosynthesis acclimation of Nannochloropsis oculata investigated by multi-wavelength chloridel fluorescence analysis. Bioresource Technology 167: 521-529.
60.Szabó, M., D. Wangpraseurt, et al. (2014). Effective light absorption and absolute electron transport rates in the coral Pocillopora damicornis. Plant Physiology and Biochemistry.
61.Tamburic, B., M. Szabó, et al. (2014). Action spectra of oxygen production and chlorophyll a fluorescience in the green microalga Nannochloropsis oculata. Bioresource Technology 169: 320-327.
62.Hakkila, K., T. Antal, et al. (2014). "Oxidative stress and photoinhibition can be separated in the cyanobacterium Synechocystis sp. PCC 6803." Biochim Biophys Acta 1837(2): 217-225.
63.Re IG OS A, M., D. Wang PR as Eur T, ETA. (2014). "spectral effects ONS YM bio low rogue photobiology studied with A programmable light engine." PL OS one 9(11): Oh112809.
64.Schreiber, U. and C. Klughammer (2013). Wavelength-dependent photodamage to Chlorella investigated with a new type of multi-color PAM chloride chlorophyll fluorometer. Photosynthesis Research 114(3): 165-177.
65.Bernát G, Schreiber U, Sendtko E, Stadnichuk IN, Rexroth S, Rögner M, Koenig F (2012) Unique Properties vs. Common Themes: The Atypical Cyanobacterium Gloeobacter violaceus PCC 7421 is Capable of State Transitions and Blue-light Induced Fluorescence Quenching.Plant & Cell Physiology: in press.
66.Schreiber U, Klughammer C, Kolbowski J (2012) Assessment of wavelength-dependent parameters of photosynthesis electron transport with a new type of Multi-Color-PAM chlorideall fluorometer. Photosynthesis Research: in press.
67.Schreiber U, Klughammer C, Kolbowski J (2011) High-end chlorophyll fluorescience analysis with the MULTI-COLOR-PAM. I. Various light qualities and their applications. PAM Application Notes, 4: 1-19.